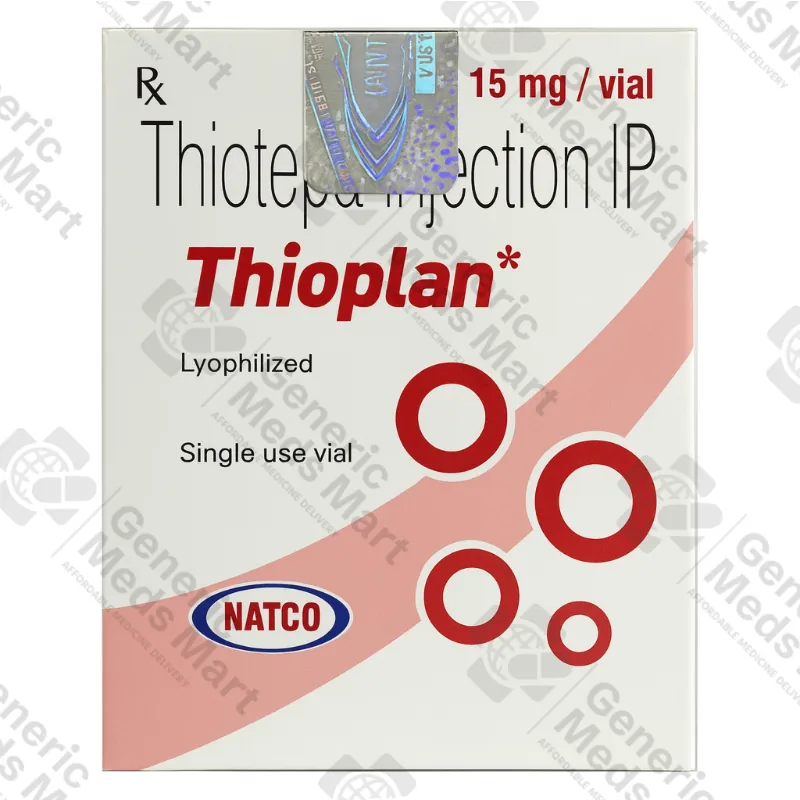
Thioplan 15 mg Thiotepa Injection (1 vial)
$599.00
1 vials in pack → $ 599.00 / vial
Thioplan 15 mg thiotepa injection (1 vial) is an alkylating cytotoxic powder for solution used in specialist chemotherapy and transplant-conditioning protocols under the supervision of experienced oncology or haematology teams. Generic Meds Mart supplies Thioplan 15 mg in original oncology packaging from licensed distributors with discreet, trackable international shipping where regulations allow.
Buy Thioplan 15 mg Thiotepa Injection Online
Thioplan 15 mg Thiotepa Injection (1 vial)
At a Glance
Generic Name: Thiotepa
Brand Name: Thioplan 15 mg
Strength & Pack Size: 15 mg thiotepa per vial, 1 single-use vial
Dosage Form & Route: Lyophilised powder for solution for IV infusion or intracavitary use as per protocol
Therapeutic Class: Alkylating agent cytotoxic chemotherapy (polyfunctional aziridine)
Primary Indications: Selected solid tumours, haematologic malignancies, intravesical therapy and conditioning for stem cell transplantation, depending on local approvals
Typical Use in Therapy: Used in combination regimens designed by transplant, haematology or oncology specialists
Mode of Action: Cross-links DNA and interferes with DNA synthesis and cell division in rapidly dividing cells
Key Benefits: Option within established high-risk or specialised regimens where thiotepa forms part of the protocol
Precautions: Strict cytotoxic handling with intensive monitoring for myelosuppression, mucositis, organ toxicity and fertility impact
Storage: Store as directed on the product carton, usually below 25 °C, protected from light and moisture in the original outer carton
Product Description
Thioplan 15 mg contains thiotepa, a well-known alkylating cytotoxic agent used in selected oncology and transplant settings. Each vial provides 15 mg of thiotepa as a lyophilised powder that must be reconstituted by trained staff before administration. Thioplan 15 mg thiotepa injection is reserved for situations where an experienced oncology, haematology or transplant team has decided that thiotepa is an appropriate component of the treatment regimen and can be delivered safely with full supportive care.
Depending on local product information and clinical practice, thiotepa injection may be used as part of conditioning regimens prior to haematopoietic stem cell transplantation, in certain solid tumours, or via intracavitary or intravesical routes for selected indications. Because thiotepa is a potent alkylating agent with predictable and sometimes profound myelosuppression and mucosal toxicity, it is not a routine outpatient medicine. Thioplan 15 mg thiotepa injection is handled exclusively in hospitals or specialist centres with the facilities to manage high-dose chemotherapy or complex multimodal regimens.
Generic Meds Mart supplies Thioplan 15 mg thiotepa injection in original, sealed oncology packaging sourced from licensed manufacturers and authorised distributors. Vials are shipped in neutral outer cartons without obvious references to cancer types or thiotepa on the shipping label, helping to preserve patient privacy. Batch numbers and expiry dates remain clearly visible on the primary pack so hospital pharmacies can document and verify each vial before inclusion in a chemotherapy protocol. Prices are shown in USD to support transparent planning for intensive or high-cost treatment courses.
Key Uses
In practice, Thioplan 15 mg thiotepa injection is used in specialised indications defined by regional approvals, product information and institutional protocols. Examples may include selected solid tumours, combination regimens for haematologic malignancies and conditioning regimens for autologous or allogeneic stem cell transplantation when thiotepa is part of an established protocol. In some settings, thiotepa may also be used by intracavitary or intravesical routes for local control of specific malignancies, always under direct specialist supervision.
The decision to use thiotepa instead of or alongside other alkylating agents, platinum compounds, anthracyclines, antimetabolites or targeted therapies is highly individualised. It depends on prior lines of therapy, organ function, performance status, anticipated transplant strategy and access to supportive care. Thioplan 15 mg thiotepa injection is never a first point of contact treatment and is only introduced once a comprehensive plan has been documented by the multidisciplinary team.
How Thiotepa Works in Chemotherapy
Thiotepa, the active ingredient in Thioplan 15 mg injection, is a polyfunctional alkylating agent of the aziridine group. After administration, thiotepa and its active metabolites can form highly reactive ethyleniminium intermediates that attack nucleophilic sites on DNA. By alkylating DNA strands, thiotepa causes intra-strand and inter-strand cross-links that interfere with replication and transcription and ultimately trigger cell death in rapidly dividing cells.
Like other alkylating agents, thiotepa is not cell cycle–specific, but its cytotoxic effects are most pronounced in tissues with high proliferative activity, such as bone marrow, gastrointestinal mucosa and many tumours. When used as part of high-dose conditioning regimens before stem cell transplantation, thiotepa contributes to intensive myeloablation and immunosuppression, which helps eradicate malignant cells and create space for engraftment of transplanted stem cells. In lower-dose or local applications, thiotepa can exert cytotoxic effects at or near the tumour site according to the treatment protocol.
Because of its broad impact on proliferating tissues, thiotepa demands experienced supportive care, including infection prophylaxis, transfusion support, nutritional management and careful monitoring of organ function. The balance between antitumour efficacy and toxicity is managed on a case-by-case basis by specialists familiar with thiotepa-based regimens.
Dosage & Administration
Thioplan 15 mg thiotepa injection must only be prescribed and administered by physicians experienced in high-dose chemotherapy, stem cell transplantation or specialised oncologic protocols. Each vial contains 15 mg thiotepa powder that must be reconstituted with a compatible diluent and, if required, further diluted in infusion fluid according to the manufacturer’s instructions and local pharmacy procedures. Vials are strictly for single use, and reconstituted solutions must be handled as cytotoxic preparations.
Doses of thiotepa are usually calculated based on body weight or body surface area and vary widely depending on indication, route of administration, combination partners and transplant strategy. Regimens may involve single or multiple doses over one or more days, often with additional conditioning agents or systemic chemotherapy. Thioplan 15 mg thiotepa injection is never self-administered; it is given intravenously, intravesically or via a controlled intracavitary route in a hospital setting where resuscitation equipment, protective isolation and experienced nursing care are available.
Patients receiving thiotepa typically require central venous access, strict fluid and electrolyte management, concurrent prophylaxis against infections and mucositis, and frequent blood tests to monitor marrow suppression and organ function. Any dose changes, delays or cessation of therapy are determined exclusively by the treating team, based on a detailed risk–benefit assessment and real-time clinical data.
Precautions
Before Thioplan 15 mg thiotepa injection is included in a regimen, the treatment team performs a comprehensive review of medical history, previous chemotherapy or radiotherapy, organ function (especially liver, kidney, heart and lungs), infection risk and fertility considerations. Thiotepa is known to cause profound myelosuppression, so patients must have frequent full blood counts, and access to transfusion support and growth factors is often required.
Thiotepa can cause significant mucosal and skin toxicity, including oral mucositis, gastrointestinal irritation and skin eruptions or hyperpigmentation, especially in high-dose or transplant-conditioning settings. Careful skin hygiene, mouth care and avoidance of prolonged skin contact with excreta containing thiotepa metabolites are advised, as directed by local protocols. Fertility may be permanently affected, and discussions about sperm banking or other fertility preservation measures typically take place before treatment. Thioplan 15 mg thiotepa injection is contraindicated in pregnancy and should not be handled or administered by pregnant staff without appropriate protective measures.
Thiotepa Side Effects
Common side effects
Common thiotepa side effects of Thioplan 15 mg thiotepa injection reflect its alkylating mechanism and impact on rapidly dividing tissues. These may include myelosuppression with neutropenia, thrombocytopenia and anaemia; fatigue; hair thinning or alopecia; nausea and vomiting; decreased appetite; oral mucositis; diarrhoea or constipation; and mild skin changes such as dryness or hyperpigmentation. Many patients report a general feeling of weakness during and after intensive cycles, which may persist until marrow and mucosal tissues recover.
With appropriate supportive care, many of these common thiotepa side effects can be anticipated and managed. Antiemetic regimens, mouthwashes, nutritional support, transfusions and growth factor support are examples of standard measures used in centres that routinely deliver thiotepa-based chemotherapy or conditioning.
Serious side effects
Serious thiotepa adverse effects of Thioplan 15 mg thiotepa injection require immediate medical attention and may be life-threatening. These can include severe and prolonged bone marrow suppression with high risk of sepsis or major bleeding; severe mucositis with inability to swallow or maintain nutrition; severe diarrhoea with dehydration; organ toxicity such as hepatic dysfunction or renal impairment; neurotoxicity in high-dose settings; and severe skin toxicity. There is also a risk of secondary malignancies with many alkylating agents when used over the long term, although this risk must be weighed against the underlying disease risk.
Patients and caregivers are instructed to contact the treatment centre urgently if there is fever, chills, uncontrolled bleeding, severe mouth pain, inability to eat or drink, reduced urine output, confusion, jaundice, severe breathlessness or any other alarming symptom during or after thiotepa treatment. The managing team may need to adjust ongoing therapy, intensify supportive care or consider alternative strategies, depending on the clinical scenario.
Storage
Thioplan 15 mg thiotepa injection vials should be stored as specified on the product carton and in the accompanying leaflet, usually below 25 °C and protected from light and moisture in the original outer carton. Vials must be kept out of reach of children and should not be used after the expiry date. Reconstituted solutions have limited stability and must be prepared and used according to local cytotoxic preparation guidelines. Unused solution, vials and administration materials should be disposed of as cytotoxic waste, following institutional and regulatory requirements.
Why Buy from Generic Meds Mart
Generic Meds Mart focuses on structured access to important oncology and transplant-supportive medicines such as Thioplan 15 mg thiotepa injection. We partner only with licensed manufacturers and authorised distributors that comply with Good Manufacturing Practice and maintain full batch traceability. Each vial of Thioplan 15 mg is supplied in sealed original packaging, allowing hospital pharmacies to verify manufacturer, strength, batch number and expiry before release to the chemotherapy or transplant unit.
By listing Thioplan 15 mg thiotepa injection in USD, Generic Meds Mart helps treatment centres and patients plan for the cost of complex regimens that may require multiple vials across several cycles. Neutral shipping cartons protect confidentiality, while tracking (where available) allows coordination between delivery and treatment scheduling. Our support team can help with ordering and logistics questions, but we do not interfere in clinical decisions; the entire responsibility for choosing thiotepa and designing regimens remains with the treating specialists.
Order Now
Thioplan 15 mg thiotepa injection is a potent hospital-only cytotoxic medicine and must never be ordered for unsupervised use. Before arranging supply through Generic Meds Mart, the patient’s oncology or transplant team should have confirmed the indication, thiotepa’s role in the regimen, the required dosing scheme, the number of vials needed per cycle and the overall schedule. They should also have verified that the centre has the infrastructure to deliver thiotepa safely, including cytotoxic handling facilities, isolation capabilities and comprehensive supportive care.
Once the plan is in place, the team or designated purchaser can calculate how many vials of Thioplan 15 mg injection are required for the initial phase of treatment. They can then select the relevant quantity on Generic Meds Mart, add it to the cart and complete secure checkout in USD. Medicines are shipped in discreet outer packaging with full original labelling inside. Patients and caregivers should never attempt to reconstitute or administer thiotepa themselves, and any concerns about side effects or dosing must be discussed promptly with the specialist team overseeing care.
FAQ about Thioplan (Thiotepa)
Q1: What is Thioplan 15 mg used for?
Thioplan 15 mg contains thiotepa, an alkylating cytotoxic agent used under specialist supervision in selected solid tumours, haematologic malignancies, intravesical or intracavitary therapy and conditioning regimens for stem cell transplantation, according to local product information and institutional protocols.
Q2: Can Thioplan 15 mg be given outside a hospital?
Thioplan 15 mg thiotepa injection is not designed for home use. It is reconstituted and administered in hospital or specialist centres with trained staff, cytotoxic preparation facilities and the ability to provide intensive monitoring and supportive care, especially when used in high-dose or transplant-conditioning regimens.
Q3: How is Thioplan 15 mg administered?
Depending on the protocol and indication, Thioplan 15 mg thiotepa injection may be administered intravenously as part of systemic chemotherapy or conditioning, or via controlled intracavitary or intravesical routes. The exact route, dose and schedule are defined by the treating team and must follow the official product information and institutional guidelines.
Q4: What monitoring is needed during thiotepa treatment?
Patients receiving Thioplan 15 mg thiotepa injection require frequent blood counts, monitoring of liver and kidney function, assessment of mucosal and skin toxicity, infection surveillance and close clinical review. In transplant settings, monitoring may be even more intensive, with detailed haemodynamic, organ function and engraftment assessments.
Q5: Can Thioplan 15 mg affect fertility?
Like many alkylating agents, thiotepa can impair fertility, and the effect may be permanent in some patients. The treating team often discusses fertility preservation options such as sperm banking before starting Thioplan 15 mg thiotepa injection, especially in younger patients or those for whom future fertility is a priority.






Reviews
There are no reviews yet.