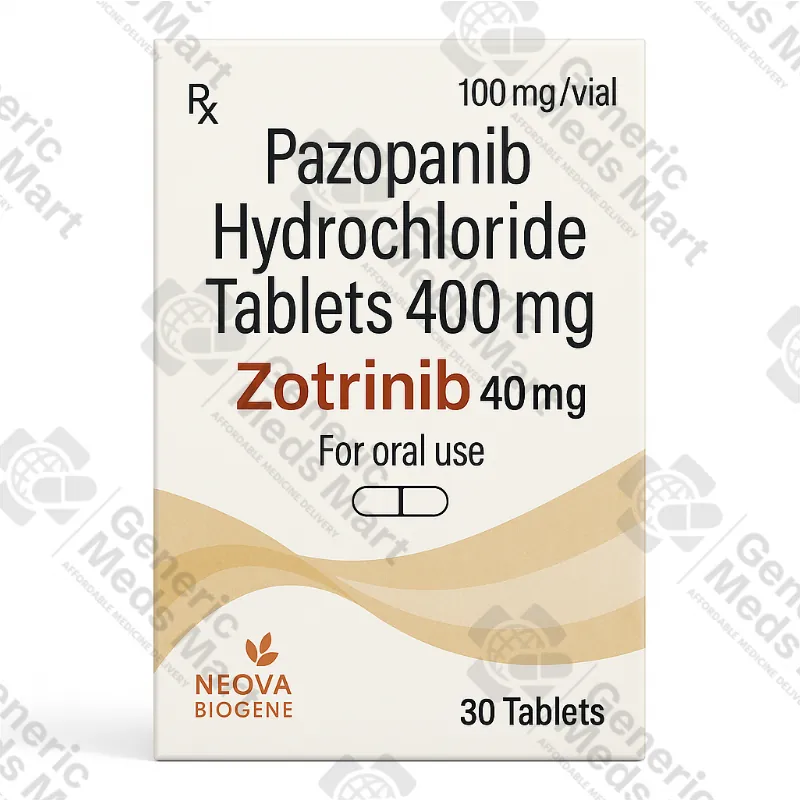
Zotrinib Pazopanib – 400 mg Tablets (1 bottle – 30 tablets)
$231.00 Original price was: $231.00.$199.00Current price is: $199.00.
30 tablets in pack → $ 6.63 / tablet
Zotrinib 400 mg pazopanib tablets (1 bottle / 30 tablets) are an oral multi-targeted tyrosine kinase inhibitor used under specialist oncology supervision for advanced or metastatic renal cell carcinoma and selected soft tissue sarcomas. Generic Meds Mart supplies Zotrinib 400 mg in original sealed oncology packaging from licensed distributors with discreet, trackable international shipping where regulations allow.
Buy Zotrinib 400 mg Pazopanib Tablets Online
Zotrinib Pazopanib – 400 mg Tablets
At a Glance
Generic Name: Pazopanib
Brand Name: Zotrinib 400 mg
Strength & Pack Size: 400 mg tablets, 1 bottle / 30 tablets
Dosage Form & Route: Oral film-coated tablets for swallowing whole
Therapeutic Class: Multi-targeted tyrosine kinase inhibitor (TKI)
Primary Indications: Advanced or metastatic renal cell carcinoma and selected soft tissue sarcomas, according to local label
Typical Use in Therapy: Continuous daily targeted therapy under oncology supervision, often after or instead of other systemic regimens
Mode of Action: Inhibits VEGFR, PDGFR and related kinases involved in tumour angiogenesis and growth
Key Benefits: Oral once-daily targeted option that can help delay disease progression in appropriately selected patients
Precautions: Requires monitoring of liver function, blood pressure, cardiac status, bleeding risk and drug interactions
Storage: Store in the original bottle below 30 °C, away from moisture and out of reach of children
Product Description
Zotrinib 400 mg contains pazopanib, an oral multi-targeted tyrosine kinase inhibitor used for advanced or metastatic renal cell carcinoma and selected soft tissue sarcomas. As a targeted therapy, Zotrinib 400 mg pazopanib tablets act on specific signalling pathways that tumours use to grow and form new blood vessels. Treatment with pazopanib is always overseen by an experienced oncology team that can assess whether this medicine fits into the overall strategy for controlling advanced disease.
Each bottle of Zotrinib 400 mg supplied by Generic Meds Mart contains 30 film-coated tablets designed to be taken by mouth once daily, at a dose and schedule defined in the official product information and by the treating oncologist. Zotrinib 400 mg is not a generic “chemotherapy pill” that can be started or stopped casually. It is part of a broader management plan that may include earlier surgery, radiotherapy, immunotherapy or other systemic treatments. Before recommending pazopanib, oncologists review tumour type, staging, prior therapies, organ function, comorbidities and potential interactions.
Generic Meds Mart provides Zotrinib 400 mg pazopanib tablets in original oncology packaging from licensed manufacturers and authorised distributors. Bottles are sealed and clearly labelled with strength, batch number and expiry date so that hospital or clinic pharmacies can verify the medicine before dispensing. Outer shipping cartons are neutral and do not mention kidney cancer, soft tissue sarcoma or pazopanib on the outside, which helps protect privacy during international delivery. Prices are displayed in USD to support long-term planning for targeted therapy that may continue for many months if well tolerated and effective.
Key Uses
Within the scope of local approvals and guidelines, Zotrinib 400 mg pazopanib tablets are used in adults with advanced or metastatic renal cell carcinoma and in certain histological subtypes of soft tissue sarcoma after prior chemotherapy. In kidney cancer, pazopanib is typically considered in patients who have unresectable or metastatic disease and are suitable for oral targeted therapy. In soft tissue sarcoma, pazopanib may be used for selected non-adipocytic tumours that have progressed after anthracycline-based regimens, where supported by evidence and regulatory status.
The place of Zotrinib 400 mg in therapy depends on what other agents are available, including tyrosine kinase inhibitors, immunotherapies and combination regimens. Oncologists may recommend pazopanib as a first-line, second-line or later-line treatment based on regional practice and prior responses. Because individual tumours behave differently, ongoing assessment with imaging, symptom review and laboratory monitoring is essential to judge whether Zotrinib 400 mg pazopanib tablets remain beneficial.
How Pazopanib Works in Chemotherapy
Pazopanib, the active ingredient in Zotrinib 400 mg, is a small-molecule tyrosine kinase inhibitor that targets several receptors involved in tumour angiogenesis and proliferation. It inhibits vascular endothelial growth factor receptors (VEGFR-1, VEGFR-2 and VEGFR-3), platelet-derived growth factor receptors (PDGFR-α and PDGFR-β), and additional tyrosine kinases such as c-Kit. By blocking these receptors, pazopanib interferes with signalling pathways that promote new blood vessel formation and support tumour survival.
In advanced renal cell carcinoma and soft tissue sarcomas, tumours often rely on angiogenesis to grow and spread. By reducing the formation of new blood vessels and altering pro-growth signalling, Zotrinib 400 mg pazopanib tablets can slow disease progression and, in some cases, shrink measurable lesions. This mechanism is different from traditional cytotoxic chemotherapy that directly damages DNA, but the end goal of controlling tumour burden is similar. Because pazopanib also affects normal vasculature and other tissues, careful monitoring is required to balance potential benefits with risks such as hypertension, bleeding or organ toxicity.
Dosage & Administration
Zotrinib 400 mg pazopanib tablets must only be prescribed and supervised by oncologists familiar with tyrosine kinase inhibitor therapy. The total daily dose is usually expressed in milligrams and composed of one or more 400 mg tablets. Tablets are generally taken once daily, at the same time each day, on an empty stomach or under specific food instructions described in the official pazopanib product information.
Patients are advised to swallow Zotrinib 400 mg tablets whole with water and not to crush, split or chew them. Because pazopanib exposure can be affected by food, antacids, proton pump inhibitors and other medicines, clear written instructions are provided to help standardise dosing conditions. Regular follow-up appointments include physical examination, laboratory tests (particularly liver function), blood pressure measurements and, where indicated, ECG or cardiac imaging. Any dose changes, temporary interruptions or permanent discontinuation of Zotrinib 400 mg are determined by the oncology team based on efficacy and tolerability.
Precautions
Before starting Zotrinib 400 mg pazopanib tablets, oncologists review the patient’s cardiovascular history, liver function, kidney function, bleeding risk, concomitant medicines and potential for drug–drug interactions. Pazopanib has been associated with hepatotoxicity, hypertension, thromboembolic events, haemorrhage, gastrointestinal perforation and, rarely, cardiac dysfunction or QT interval prolongation. Baseline tests and regular monitoring help detect emerging toxicity early.
Patients are usually advised to report symptoms such as severe fatigue, yellowing of the skin or eyes, dark urine, right-sided upper abdominal pain, new or worsening headaches, chest pain, palpitations, shortness of breath, sudden leg swelling, unusual bruising or bleeding, or severe abdominal pain. Pazopanib can interact with medicines that affect CYP3A4 and certain transporters, so all prescription medicines, over-the-counter drugs and herbal supplements should be reviewed before and during therapy. Zotrinib 400 mg pazopanib tablets are generally avoided during pregnancy and breastfeeding, and effective contraception is usually recommended during treatment and for a period afterwards.
Pazopanib Side Effects
Common side effects
Common pazopanib side effects seen with Zotrinib 400 mg include diarrhoea, nausea, vomiting, decreased appetite, changes in taste, fatigue, weight loss, hair colour lightening, skin depigmentation, mild rash, hand–foot skin reactions, hypertension, and mild to moderate liver enzyme elevations. Some patients also experience hypothyroidism, mild proteinuria or transient changes in blood counts.
Many of these common pazopanib side effects can be managed with supportive medicines, dietary adjustments, local skin care, blood pressure control and, when necessary, dose reductions or temporary interruptions. The oncology team will provide guidance on monitoring blood pressure at home, recognising early signs of dehydration and managing diarrhoea. Regular blood tests help identify liver or thyroid changes that may need intervention.
Serious side effects
Serious pazopanib adverse effects require urgent medical attention and may lead to permanent discontinuation of Zotrinib 400 mg. These can include severe hepatotoxicity with marked liver enzyme elevations or jaundice, hypertensive crises, serious bleeding events, venous or arterial thromboembolism, heart failure, QT interval prolongation with arrhythmia, gastrointestinal perforation or fistula, and severe skin reactions.
Patients should seek emergency care if they develop intense right upper abdominal pain, pronounced jaundice, severe headaches with visual disturbances, chest pain, sudden shortness of breath, coughing up blood, one-sided leg swelling, rapid weight gain with swelling, fainting episodes, black or bloody stools or any other alarming symptom. The treating team may need to stop Zotrinib 400 mg pazopanib tablets, address the complication and reassess whether further targeted therapy is appropriate.
Storage
Zotrinib 400 mg pazopanib tablets should be stored in the original bottle with the cap tightly closed, below the temperature specified in the product information, usually not above 30 °C. The bottle should be kept dry, away from direct heat and light, and out of the sight and reach of children. Tablets should not be transferred into unlabelled containers or pill organisers without clear agreement from the treating team, as this may compromise identification and stability. Any tablets remaining after the expiry date should not be used and should be disposed of according to local guidance for cytotoxic or targeted cancer medicines.
Why Buy from Generic Meds Mart
Generic Meds Mart is focused on structured access to targeted oncology medicines such as Zotrinib 400 mg pazopanib tablets. We work only with licensed manufacturers and authorised distributors that adhere to Good Manufacturing Practice and provide full batch traceability. Each bottle of Zotrinib 400 mg supplied by Generic Meds Mart is delivered in sealed original packaging, so oncology pharmacies can verify brand, strength, batch number and expiry before dispensing.
By listing Zotrinib 400 mg pazopanib tablets in USD, Generic Meds Mart helps patients and clinics plan the cost of long-term targeted therapy more transparently. Neutral shipping cartons protect privacy while still allowing tracked international delivery where regulations permit. Our role is limited to supply and logistics; we do not provide medical advice or replace the treating oncologist. All decisions about starting, continuing, adjusting or stopping Zotrinib 400 mg remain strictly with your oncology team.
Order Now
Zotrinib 400 mg pazopanib tablets are a potent targeted therapy for advanced cancers and must never be used without a detailed treatment plan from an experienced oncologist. Before ordering from Generic Meds Mart, patients and clinicians should confirm the diagnosis, prior treatments, intended pazopanib dose, monitoring schedule and criteria for continuing or adjusting therapy.
Once a plan is agreed, the number of bottles of Zotrinib 400 mg (1 bottle / 30 tablets) required for the initial treatment period can be calculated. You can then select the needed quantity on Generic Meds Mart, add it to your cart and complete secure checkout in USD. Medicines are dispatched in discreet packaging with full original labelling inside. Patients should not change the dose, interrupt therapy or restart Zotrinib 400 mg on their own; any change in treatment or any serious new symptom must be discussed promptly with the oncology team.
FAQ about Zotrinib (Pazopanib)
Q1: What is Zotrinib 400 mg used for?
Zotrinib 400 mg contains pazopanib, a multi-targeted tyrosine kinase inhibitor used under specialist supervision for advanced or metastatic renal cell carcinoma and certain soft tissue sarcomas, in line with local product information and guidelines.
Q2: Is Zotrinib 400 mg chemotherapy or targeted therapy?
Zotrinib 400 mg pazopanib tablets are considered targeted therapy rather than traditional cytotoxic chemotherapy. They block specific kinases involved in tumour angiogenesis and growth. However, they are still powerful anti-cancer medicines with important side effects and monitoring requirements.
Q3: How long will I need to stay on Zotrinib 400 mg?
Duration of Zotrinib 400 mg pazopanib treatment varies. Many patients continue therapy as long as the disease remains controlled and side effects are manageable. Treatment may be changed or stopped if the tumour progresses or toxicity becomes unacceptable. Your oncology team will review scans, symptoms and laboratory results regularly to decide how long to continue pazopanib.
Q4: Can I take other medicines while using Zotrinib 400 mg?
Some medicines and supplements can interact with pazopanib, particularly those affecting CYP3A4 or gastric pH. Always give your oncology team and pharmacist a complete list of prescription drugs, over-the-counter products and herbal remedies before and during therapy. Never start or stop another medicine without checking for interactions with Zotrinib 400 mg.
Q5: What monitoring do I need on Zotrinib 400 mg?
Patients taking Zotrinib 400 mg pazopanib tablets usually need regular liver function tests, blood pressure checks, urine tests, sometimes ECGs and periodic imaging to assess response. These checks help detect side effects such as hepatotoxicity, hypertension, cardiac changes or proteinuria early, so that doses can be adjusted and complications managed promptly.







Reviews
There are no reviews yet.