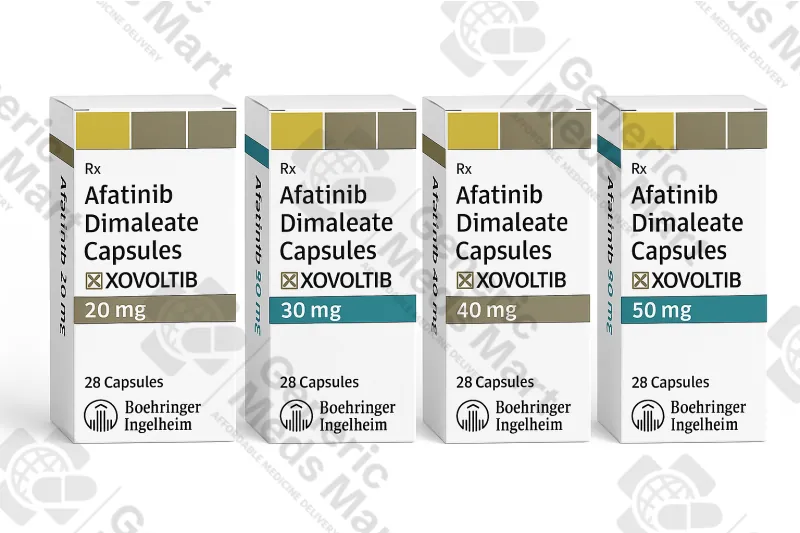
Xovoltib Afatinib – 20 mg, 30 mg, 40 mg, 50 mg Capsules
$1,899.00 – $2,292.00Price range: $1,899.00 through $2,292.00
Xovoltib afatinib 20 mg, 30 mg, 40 mg and 50 mg capsules (1 pack / 28 caps) are oral EGFR / ErbB tyrosine kinase inhibitor therapy used under specialist oncology supervision for advanced or metastatic EGFR mutation–positive non-small cell lung cancer. Generic Meds Mart supplies Xovoltib in original manufacturer packaging from licensed distributors with discreet, trackable international delivery where regulations allow.
Buy Xovoltib Afatinib 20 mg, 30 mg, 40 mg and 50 mg Capsules Online
Xovoltib Afatinib – 20 mg, 30 mg, 40 mg, 50 mg Capsules
At a Glance
Generic Name: Afatinib
Brand Name: Xovoltib
Strength & Pack Size: 20 mg, 30 mg, 40 mg and 50 mg capsules, 1 pack / 28 capsules for each strength
Dosage Form & Route: Oral capsules swallowed whole, usually once daily on an empty stomach
Therapeutic Class: Irreversible EGFR / ErbB family tyrosine kinase inhibitor (targeted therapy)
Primary Indications: Advanced or metastatic EGFR mutation–positive non-small cell lung cancer (NSCLC) and other approved NSCLC settings per local label
Typical Use in Therapy: First-line or subsequent-line targeted therapy in patients with confirmed sensitising EGFR mutations under specialist oncology supervision
Mode of Action: Irreversibly blocks signalling in EGFR and related ErbB receptors that drive tumour cell growth and survival
Key Benefits: Oral, mutation-focused treatment option with defined dosing and monitoring strategies for eligible patients
Precautions: Diarrhoea, rash, stomatitis, lung toxicity, liver enzyme elevations and cardiac or ocular effects require close monitoring
Storage: Store in original packaging below 30 °C, protected from moisture and out of reach of children
Product Description
Xovoltib contains afatinib, an oral, irreversible EGFR / ErbB family tyrosine kinase inhibitor used as targeted therapy for patients with advanced or metastatic non-small cell lung cancer (NSCLC) whose tumours harbour specific EGFR activating mutations, according to local approvals and guidelines. Instead of traditional intravenous chemotherapy, Xovoltib afatinib is taken by mouth, usually once daily, making it possible for many patients to receive systemic targeted therapy at home while being monitored closely by their oncology team.
The Xovoltib range includes 20 mg, 30 mg, 40 mg and 50 mg strengths, all supplied as 1 pack / 28 capsules. These strengths allow oncologists to initiate therapy at a recommended starting dose for eligible patients and then adjust the dose up or down in structured steps depending on side effects and tolerability. The 28-capsule pack format aligns with common once-daily treatment cycles, roughly corresponding to four weeks of continuous therapy at a given dose.
Afatinib targets signalling within the EGFR (ErbB1), HER2 (ErbB2) and other members of the ErbB family, which are often dysregulated in EGFR mutation–positive NSCLC. By inhibiting these receptors, Xovoltib can help slow or shrink tumours that depend on EGFR-driven pathways. It does not replace surgery, radiotherapy or other systemic therapies, but rather forms part of an integrated treatment strategy for advanced disease in appropriately selected patients with documented EGFR mutations.
Generic Meds Mart supplies Xovoltib afatinib capsules only in original manufacturer packaging sourced from licensed distributors. Each pack shows the strength, generic name, batch number and expiry date, enabling hospital and clinic pharmacies to verify product authenticity and traceability. Neutral outer packaging is used for shipping, so there is no external reference to lung cancer, oncology or targeted therapy on the parcel. Our role is strictly limited to structured access and logistics; all treatment decisions remain with your oncology team.
Key Uses
According to local approvals, molecular testing and oncology guidelines, Xovoltib afatinib capsules are generally used for:
- Advanced or metastatic EGFR mutation–positive NSCLC, particularly tumours with defined sensitising EGFR mutations in treatment-naïve patients or selected subsequent-line settings.
- Selected other EGFR-related NSCLC indications where afatinib has a recognised role in regional product information or protocols.
Your oncologist will confirm whether your tumour carries the relevant EGFR mutations and whether Xovoltib is an appropriate targeted therapy option based on your disease stage, prior treatments, comorbidities and overall treatment plan.
How Afatinib Works in Chemotherapy
Afatinib, the active ingredient in Xovoltib capsules, is an irreversible inhibitor of the ErbB family of receptors, including EGFR (ErbB1), HER2 (ErbB2) and ErbB4. By forming covalent bonds with these tyrosine kinase domains, afatinib blocks downstream signalling pathways that promote tumour cell proliferation, survival, angiogenesis and metastasis. This mechanism is particularly relevant in tumours driven by activating EGFR mutations, where continuous signalling through EGFR is a key driver of disease.
By shutting down aberrant EGFR / ErbB signalling, Xovoltib afatinib can slow tumour growth and, in many patients, induce measurable tumour shrinkage or disease stabilisation. However, as with other targeted therapies, resistance may eventually develop through additional mutations or bypass pathways. For this reason, ongoing imaging, clinical evaluation and, where appropriate, repeat molecular testing form part of long-term management. Afatinib is not a cure for advanced NSCLC, but it is an important option within the broader set of EGFR-directed therapies when used according to up-to-date guidelines.
Dosage & Administration
Xovoltib afatinib dosing is determined by oncology specialists and should follow local prescribing information. Many patients begin treatment at a standard once-daily dose based on the 20 mg, 30 mg or 40 mg strengths, with potential adjustments depending on side effects, organ function and individual clinical factors. Dose reductions often involve stepwise decreases using the available strengths of 50 mg, 40 mg, 30 mg and 20 mg to maintain efficacy while improving tolerability.
Xovoltib capsules are usually taken once daily at approximately the same time, on an empty stomach, either at least one hour before or two to three hours after food, as food can influence afatinib absorption. Capsules should be swallowed whole with water and must not be opened, crushed or chewed. If a dose is missed, patients should follow the instructions from their oncology team rather than taking extra capsules to “catch up”. Any dose changes, temporary interruptions or restarts must be directed by the treating oncologist and should never be made independently.
Precautions
Before and during treatment with Xovoltib afatinib capsules, careful monitoring is required. Diarrhoea is a very common side effect and can become severe or even life-threatening if it leads to dehydration, electrolyte disturbance or kidney injury. Patients are usually counselled to start antidiarrhoeal medication promptly at the first sign of loose stools and to increase fluid intake, with rapid contact to the clinic if diarrhoea worsens or persists.
Skin reactions, including acneiform rash, dryness, fissures and nail changes such as paronychia, are also common and may need dermatologic supportive care and, in some cases, dose modification. Xovoltib can cause stomatitis (soreness or ulcers in the mouth), eye irritation, elevations in liver enzymes and, more rarely, interstitial lung disease (ILD) or pneumonitis. Any new or worsening shortness of breath, cough or fever must be reported immediately, as ILD can be serious and may require permanent discontinuation of afatinib.
Baseline and periodic liver function tests, renal function, cardiac status and, if indicated, ophthalmologic assessments may be recommended, particularly in patients with pre-existing conditions. Afatinib can harm an unborn baby, so effective contraception is necessary for people who can become pregnant and for partners, as advised by the oncology team. Breastfeeding is generally not recommended while taking Xovoltib afatinib capsules. Drug interactions with P-glycoprotein modulators and other medicines should be reviewed carefully before and during therapy.
Afatinib Side Effects
Common side effects
Common afatinib side effects reported with Xovoltib capsules include diarrhoea, rash, dry or itchy skin, acneiform eruptions, nail changes, stomatitis or mouth ulcers, decreased appetite, nausea, vomiting, abdominal pain, weight loss, conjunctivitis or eye irritation, and mild increases in liver enzymes on blood tests. Fatigue and general weakness are also frequently noted, especially during the first weeks of therapy or after dose changes.
These common afatinib side effects can often be managed with early intervention, supportive medications, skin and nail care, antidiarrhoeal regimens and dietary adjustments, combined with dose interruptions or reductions when needed. Patients should be encouraged to report symptoms promptly rather than waiting for routine visits, so that the oncology team can respond quickly and prevent complications.
Serious side effects
Serious afatinib adverse effects require urgent medical assessment and may include severe or persistent diarrhoea with signs of dehydration, severe skin reactions with blistering or widespread rash, signs of interstitial lung disease such as new or worsening shortness of breath, cough and fever, marked liver injury with jaundice, dark urine and right upper abdominal pain, severe eye pain or sudden changes in vision, and significant cardiac symptoms such as chest pain, palpitations or syncope.
If any of these warning signs occur during Xovoltib afatinib treatment, patients must seek emergency medical care and inform their oncology team as soon as possible. The medicine may need to be interrupted or permanently discontinued, and appropriate investigations and supportive treatments should be initiated promptly.
Storage
Xovoltib afatinib capsules should be stored in their original blister or bottle at the temperature specified in the package leaflet, generally below 30 °C, protected from moisture and direct heat. The pack should remain tightly closed when not in use, and capsules should be removed only immediately before dosing. As with all potent oncology medicines, Xovoltib must be kept out of the sight and reach of children and stored separately from non-prescription household medicines. Unused or expired capsules should be returned to a pharmacy or disposed of in accordance with local guidance for hazardous or cytotoxic medicines, not in household waste.
Why Buy from Generic Meds Mart
Generic Meds Mart focuses on structured access to modern targeted oncology medicines such as Xovoltib afatinib capsules. We work only with licensed manufacturers and authorised distributors who follow Good Manufacturing Practice standards and maintain traceable batch records. Each pack of Xovoltib 20 mg, 30 mg, 40 mg or 50 mg is supplied in original sealed packaging, with clearly visible strength, batch number and expiry date so oncology clinics and pharmacies can verify authenticity before dispensing.
Displaying prices in USD and listing all available strengths helps patients and clinics plan targeted therapy over multiple months and adjust quantities as dosing changes. Neutral outer packaging and trackable delivery options, where regulations allow, support privacy and make it easier to coordinate supply with clinic visits and monitoring. Our role is strictly limited to sourcing and logistics; we do not provide medical advice and do not replace your oncology team in deciding if Xovoltib afatinib therapy is appropriate.
Order Now
Xovoltib afatinib 20 mg, 30 mg, 40 mg and 50 mg capsules are potent targeted agents that must only be prescribed and monitored by experienced oncology professionals. Before arranging supply through Generic Meds Mart, ensure that your tumour’s EGFR mutation status has been confirmed, your overall treatment strategy has been discussed in detail with your oncologist, and a clear afatinib dosing and monitoring plan is in place.
Once your plan is agreed, your clinic or designated purchaser can calculate how many packs of each Xovoltib strength are needed to cover the upcoming treatment interval, including any anticipated dose adjustments. The required strengths – 20 mg, 30 mg, 40 mg and 50 mg (1 pack / 28 caps) – can then be selected on Generic Meds Mart, added to the cart and ordered via secure checkout in USD. Medicines will be dispatched in discreet packaging. Any new or worsening symptoms or concerns while taking Xovoltib afatinib must be reported promptly to your oncology team; patients should never alter or stop therapy on their own.
FAQ about Xovoltib (Afatinib)
Q1: What type of lung cancer is Xovoltib used for?
Xovoltib contains afatinib, an oral EGFR / ErbB family tyrosine kinase inhibitor used mainly in advanced or metastatic non-small cell lung cancer (NSCLC) where the tumour carries specific sensitising EGFR mutations, in line with local approvals and guidelines.
Q2: How is Xovoltib different from traditional chemotherapy?
Xovoltib afatinib is a targeted therapy rather than traditional cytotoxic chemotherapy. It focuses on EGFR and related ErbB receptors that drive tumour growth in EGFR mutation–positive NSCLC. It is taken orally once daily, but side effects can still be significant and require careful monitoring, just like chemotherapy.
Q3: How long will I need to take Xovoltib?
The duration of Xovoltib afatinib treatment varies between patients. Many remain on therapy as long as scans show disease control and side effects remain manageable. If disease progresses or side effects become unacceptable despite dose adjustments, your oncologist may recommend switching to another treatment.
Q4: Can I manage Xovoltib side effects at home?
Many side effects, such as mild diarrhoea, rash and mouth soreness, can be managed at home with supportive care and medicines prescribed by your oncology team. However, you should report symptoms early, follow instructions closely and seek urgent help if diarrhoea becomes severe, breathing changes, jaundice, chest pain or other alarming signs appear.
Q5: Do I need special tests while on Xovoltib?
Patients on Xovoltib afatinib capsules usually need regular blood tests (including liver and kidney function), periodic imaging to assess tumour response, and close monitoring of diarrhoea, skin changes and respiratory symptoms. Your oncologist may also review cardiac status, blood pressure and eye health, depending on your overall risk profile and any co-existing conditions.
Q6: Is Xovoltib the same as Gilotrif (Generic Gilotrif)?
Xovoltib is Generic Gilotrif — it contains the same active ingredient (afatinib) as Gilotrif. It’s used for the same purpose at the same strength, while differences may be in manufacturer, inactive ingredients (excipients), tablet appearance, and packaging. If you’re switching between products, confirm the substitution with a qualified healthcare professional.






Darrel –
I’m so glad i finally find reliable source of my medicine! Thanks