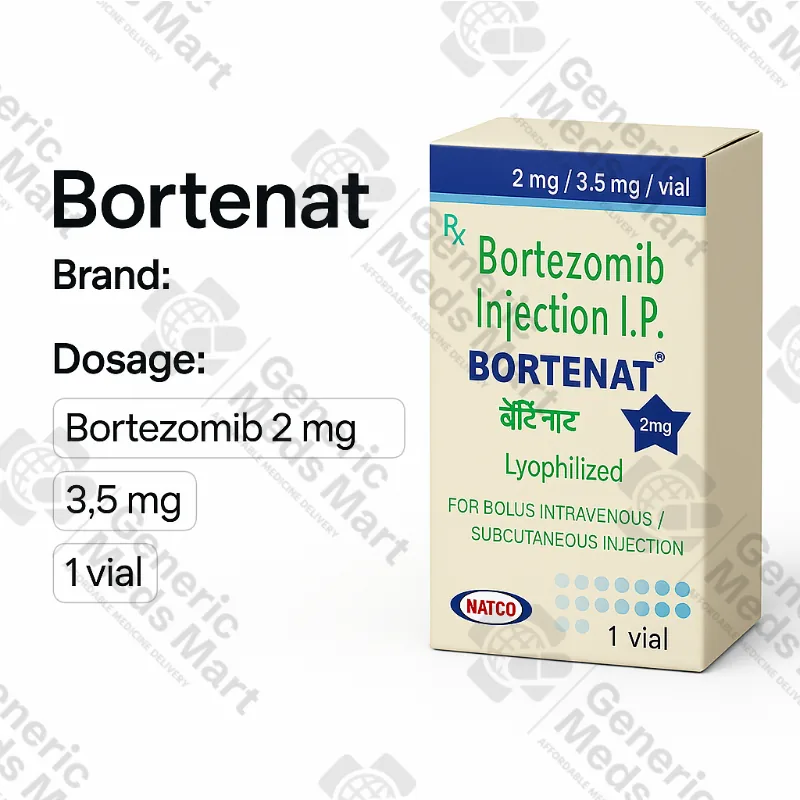
Bortenat 2 mg, 3.5 mg Bortezomib Injection
$135.00 – $199.00Price range: $135.00 through $199.00
Bortenat is a bortezomib-based injectable chemotherapy supplied as 2 mg and 3.5 mg single-use vials of lyophilised powder for solution for IV or subcutaneous injection in multiple myeloma and mantle cell lymphoma. Generic Meds Mart provides Bortenat 2 mg and 3.5 mg vials in original oncology cartons from licensed distributors with discreet, trackable international shipping where regulations allow.
Buy Bortenat 2 mg and 3.5 mg Bortezomib Injection Vials Online
Bortenat 2 mg and 3.5 mg Bortezomib Injection Vials
At a Glance
Generic Name: Bortezomib
Brand Name: Bortenat
Strength & Pack Size: 2 mg and 3.5 mg single-use vials of bortezomib lyophilised powder for solution for injection
Dosage Form & Route: Lyophilised powder for IV or subcutaneous injection after reconstitution
Therapeutic Class: Proteasome inhibitor antineoplastic agent
Primary Indications: Multiple myeloma and mantle cell lymphoma, according to approved protocols
Usual Adult Dose: Body-surface-area–based dose (mg/m²) on specific days of each cycle under specialist supervision
Prescription Status: Prescription-only cytotoxic medicine for hospital or infusion clinic use
Storage: Store below 25 °C in the original carton, protect from light and follow local cytotoxic handling procedures
Product Description
Bortenat is a bortezomib-based injectable chemotherapy used in the treatment of multiple myeloma and mantle cell lymphoma under the supervision of experienced haematology and oncology specialists. Each Bortenat vial contains bortezomib as a lyophilised powder that must be reconstituted with an appropriate diluent before use, then administered as either an intravenous or subcutaneous injection according to protocol. The availability of both 2 mg and 3.5 mg vial strengths allows clinicians to tailor total bortezomib doses based on body surface area, treatment phase, combination partners and individual tolerability.
Bortezomib was the first proteasome inhibitor to become widely used in clinical practice and remains a backbone of many first-line and relapsed multiple myeloma regimens. It is also used in selected patients with mantle cell lymphoma, often as part of combination regimens. Bortenat provides a generic bortezomib option that is intended to deliver comparable quality and activity when used correctly within established protocols. Because bortezomib is a potent cytotoxic agent, Bortenat vials are strictly hospital-use medicines and are never self-administered by patients at home.
Generic Meds Mart supplies Bortenat 2 mg and 3.5 mg vials in original oncology cartons from licensed distributors. Each vial is clearly labelled with strength, generic name, batch number, expiry date and storage conditions so that pharmacy and chemotherapy teams can verify every unit before reconstitution. Orders are shipped in neutral outer packaging that does not reference myeloma, lymphoma or chemotherapy, helping to protect privacy while still enabling traceability and documentation for hospital records. Our role is to support access and logistics; all clinical decisions about bortezomib belong to your treating specialists.
Key Uses
Bortenat is used primarily in adults with multiple myeloma, either as part of initial therapy or in relapse settings, depending on local guidelines and previous treatment history. Bortezomib-based regimens may include combinations with immunomodulatory agents, corticosteroids, alkylating agents and other drugs, and may be used before or after stem cell transplantation depending on the overall treatment strategy. The aim is to reduce the burden of abnormal plasma cells in the bone marrow, relieve symptoms such as bone pain, anaemia and infections, and extend progression-free and overall survival.
In mantle cell lymphoma, Bortenat may be used in specific lines of therapy for patients who are suitable for bortezomib-based regimens, often as part of multi-agent protocols. Patient selection is based on disease stage, comorbidities, organ function, performance status and prior therapies. Bortezomib is not appropriate for every patient with myeloma or mantle cell lymphoma; your haematology team will decide if Bortenat fits into your treatment plan after reviewing all diagnostic and clinical details.
How Bortezomib Works in Chemotherapy
Bortezomib, the active ingredient in Bortenat, is a reversible inhibitor of the 26S proteasome, a protein complex responsible for degrading ubiquitinated proteins inside cells. Inhibition of the proteasome leads to accumulation of regulatory proteins and misfolded proteins, disrupting normal cell signalling, cell cycle control and survival pathways. Myeloma cells and some lymphoma cells are particularly dependent on a functioning proteasome and protein quality control system, so they can be more vulnerable to proteasome inhibition than many normal cells.
By blocking proteasome activity, bortezomib can trigger stress pathways, inhibit NF-κB signalling and promote programmed cell death in malignant plasma cells and certain lymphoma cells. At the same time, proteasome inhibition can affect normal cells and contribute to side effects such as neuropathy and cytopenias. For this reason, careful dosing schedules with rest periods, vigilant monitoring and dose adjustments are built into bortezomib treatment protocols to maximise anti-tumour effects while managing toxicity.
Dosage & Administration
Bortenat dosing is based on body surface area and must always follow the specific regimen chosen by your haematology or oncology team. Bortezomib may be administered intravenously as a rapid bolus injection or subcutaneously into the thigh or abdomen, depending on the protocol and individual tolerance. Standard schedules often involve injections on specific days of a 21-day or 28-day cycle, for example on days 1, 4, 8 and 11, followed by a rest period. The total dose per injection, expressed in mg/m², is calculated from your height and weight by the treating team.
Bortenat vials must be reconstituted with the recommended diluent by trained staff using aseptic technique in a suitable preparation area. The resulting solution is used promptly or stored according to the product information and institutional guidelines. Patients are monitored during and after injections for blood pressure, infusion or injection reactions, neuropathy symptoms and other side effects. The regimen may be combined with other agents such as dexamethasone, lenalidomide, cyclophosphamide or other chemotherapy drugs, and the full schedule is always defined by a specialist. Patients should never attempt to handle, reconstitute or inject Bortenat themselves.
Precautions
Before starting Bortenat, your haematology team will review your medical history, cardiovascular status, peripheral nerve function, liver and kidney function, blood counts, infection history and concomitant medicines. Bortezomib can cause or worsen peripheral neuropathy, so pre-existing neuropathy is an important factor in deciding dose and route of administration. Blood pressure, heart function and fluid balance are monitored closely, especially in patients with a history of heart failure or cardiac disease.
Bortezomib may lower white blood cell and platelet counts, increasing the risk of infections and bleeding. Regular full blood counts are essential, and dose modifications or treatment breaks may be required if significant cytopenias occur. Because herpes zoster reactivation is more common in patients receiving bortezomib, antiviral prophylaxis may be recommended as part of your regimen. Effective contraception is usually required during treatment and for a period afterwards, as bortezomib could potentially harm a developing baby. Bortenat is not intended for use in pregnancy or breastfeeding unless the benefit clearly outweighs the risk and this is carefully documented by your specialist.
Bortezomib Side Effects
Common side effects
Common bortezomib side effects include fatigue, weakness, nausea, vomiting, diarrhoea or constipation, decreased appetite, mild neuropathy with tingling or numbness in hands and feet, low blood counts, infections of the upper respiratory tract, mild shortness of breath and injection site reactions such as redness, bruising or discomfort. Many patients experience some degree of thrombocytopenia or neutropenia during treatment, which is monitored using regular blood tests. In many cases, these effects can be managed through supportive medication, dose adjustments, schedule changes and careful monitoring by the treatment team.
Serious side effects
Serious bortezomib adverse effects require urgent medical attention and may lead to dose reduction or discontinuation of therapy. These include severe or rapidly worsening peripheral neuropathy affecting daily function, severe infections or sepsis, marked thrombocytopenia with significant bleeding or bruising, severe neutropenia with fever, heart failure or new or worsening shortness of breath, serious gastrointestinal complications such as ileus or perforation, and rare but serious liver or lung toxicity. Any chest pain, sudden shortness of breath, pronounced leg swelling, confusion, severe abdominal pain, high fever or other alarming symptom during Bortenat therapy should be reported immediately to your haematology team or emergency services.
Storage
Unopened Bortenat vials should be stored below 25 degrees Celsius, protected from light and moisture, in their original cartons until the time of reconstitution. Vials should not be frozen. After reconstitution, the bortezomib solution has limited stability and must be handled and stored according to the product information and institutional cytotoxic guidelines, typically by the hospital pharmacy or chemotherapy unit. Patients are not expected to store or transport reconstituted vials themselves. Used vials, syringes and materials must be disposed of as cytotoxic waste in accordance with local regulations and hospital policies.
Why Buy from Generic Meds Mart
Generic Meds Mart helps clinics and patients access Bortenat bortezomib injection in regions where availability or pricing may otherwise limit treatment choices. We source Bortenat 2 mg and 3.5 mg vials only from licensed manufacturers and authorised distributors who comply with recognised quality standards and maintain traceable batch and expiry records. By supplying vials in sealed, original oncology cartons, we support hospital pharmacies in verifying that each vial matches the prescribed strength and product name before it is added to chemotherapy protocols.
All orders placed through Generic Meds Mart are priced in USD and processed via secure online checkout. Parcels are prepared in neutral outer packaging with no visible references to myeloma, lymphoma, bortezomib or chemotherapy, which can make shipping and receipt more discreet. Where available, tracked shipping options allow haematology teams to plan treatment cycles around anticipated delivery dates and reduce the risk of delays due to supply interruptions. While we facilitate access and logistics, all decisions about whether bortezomib is appropriate, how it should be dosed and how long therapy should continue remain entirely with your specialist team.
Order Now
Before you order Bortenat online from Generic Meds Mart, you should have a confirmed diagnosis of multiple myeloma or mantle cell lymphoma and a written treatment plan from your haematologist or oncologist that specifically includes bortezomib. Your specialist will determine whether Bortenat fits into your therapy sequence, what total dose and schedule you require, whether IV or subcutaneous administration is preferred and how many cycles are planned. Based on this plan, your clinic can calculate how many Bortenat 2 mg and 3.5 mg vials are needed per cycle and for the overall course.
You should never attempt to self-prescribe bortezomib, adjust your dose on your own or substitute between different vial strengths without direct guidance from your treating team. If you develop new or worsening symptoms such as severe neuropathy, bleeding, persistent fever, chest pain, breathing difficulty or sudden swelling while receiving Bortenat, contact your haematology clinic or emergency services immediately. Generic Meds Mart exists to help ensure that the bortezomib specified in your regimen is available in a timely, verifiable and discreet way, while the complex clinical management of your cancer stays with your specialists.
FAQ about Bortenat (Bortezomib)
Q1: What conditions is Bortenat used to treat?
Bortenat contains bortezomib, a proteasome inhibitor used mainly in adults with multiple myeloma and in selected patients with mantle cell lymphoma. Your haematology team decides when bortezomib is appropriate based on disease stage, prior treatments, overall health and current treatment guidelines.
Q2: Is Bortenat given as an IV drip or an injection under the skin?
Bortenat can be given either as an intravenous bolus injection or as a subcutaneous injection, depending on the chosen protocol and your individual tolerance. Subcutaneous administration is often preferred in many patients because it can reduce certain side effects, especially neuropathy, but the route is always determined by your specialist.
Q3: Can I receive Bortenat treatment at home?
Bortezomib injections must be prepared and administered by trained healthcare professionals in a hospital or clinic setting with appropriate monitoring and emergency support. Bortenat is not intended for self-injection or home administration. Some patients may be treated in outpatient infusion centres, but preparation and injection are still handled by clinical staff.
Q4: What monitoring will I need while on Bortenat?
During Bortenat treatment you will have regular blood tests to check white blood cells, red cells, platelets, kidney and liver function, as well as assessments for neuropathy, blood pressure and any signs of infection or heart problems. Your team may also monitor weight, fluid status and, when appropriate, imaging or disease markers to assess response. These results guide dose adjustments and decisions about continuing or changing therapy.
Q5: Will Bortenat cure my myeloma or lymphoma?
Bortezomib is an effective component of many regimens and can produce deep and sometimes long-lasting responses, but multiple myeloma and mantle cell lymphoma are generally considered chronic or relapsing conditions. In most cases, Bortenat is used to control disease, extend remission and improve quality of life rather than as a guaranteed cure. Your haematology team will discuss realistic goals of therapy in your individual situation.
Be the first to review “Bortenat 2 mg, 3.5 mg Bortezomib Injection” Cancel reply
Related products
Anticancer





Reviews
There are no reviews yet.