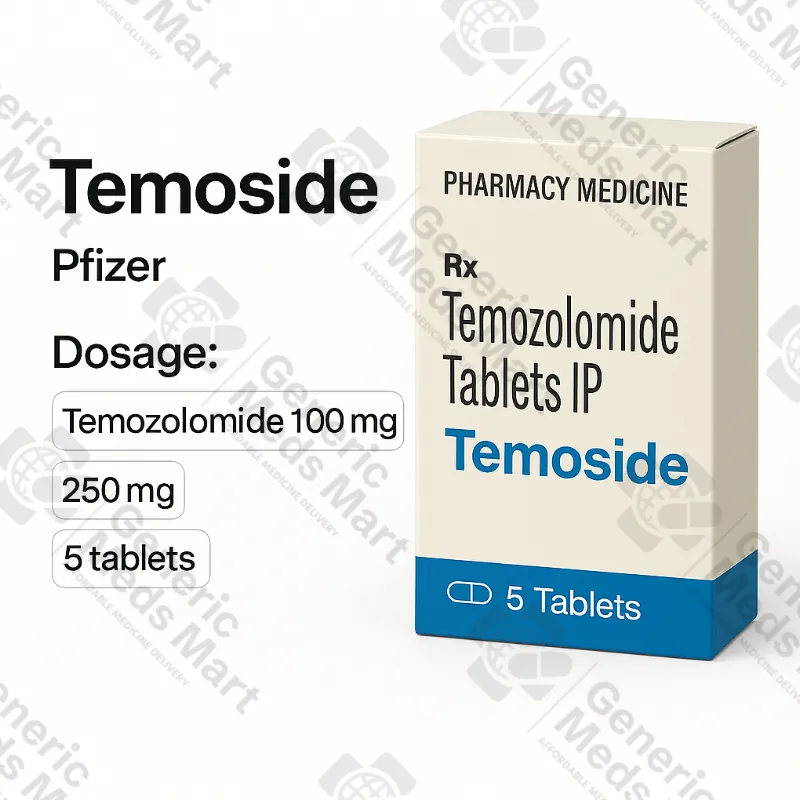
Temoside Temozolomide – 100 mg, 200 mg Tablets (1 pack / 5 tabs)
$72.00 – $108.00Price range: $72.00 through $108.00
Temoside 100 mg and 200 mg temozolomide tablets (1 pack / 5 tabs) are oral alkylating chemotherapy used under specialist neuro-oncology supervision for high-grade glioma, glioblastoma multiforme and anaplastic astrocytoma in concurrent and adjuvant chemoradiation or recurrence settings. Generic Meds Mart supplies Temoside in original oncology packaging from licensed manufacturers with discreet, trackable international delivery where regulations allow.
Buy Temoside 100 mg and 200 mg Temozolomide Tablets Online
Temoside Temozolomide – 100 mg, 200 mg Tablets
At a Glance
Generic Name: Temozolomide
Brand Name: Temoside
Strength & Pack Size: 100 mg and 200 mg tablets, 1 pack / 5 tablets for each strength
Dosage Form & Route: Oral tablets for swallowing whole on an empty stomach
Therapeutic Class: Oral alkylating cytotoxic chemotherapy
Primary Indications: High-grade glioma, glioblastoma multiforme and anaplastic astrocytoma according to local protocols
Typical Use in Therapy: Concurrent and adjuvant chemoradiation, and treatment of recurrent high-grade brain tumours under neuro-oncology supervision
Mode of Action: Causes DNA damage in tumour cells through methylation of guanine bases, triggering cell death
Key Benefits: Well-established oral component of glioblastoma treatment that can be delivered at home with careful monitoring
Precautions: Requires close monitoring of blood counts, infection risk, liver function and nausea control
Storage: Store in original blister below 25–30 °C, protected from moisture, heat and children
Product Description
Temoside contains temozolomide, an oral alkylating chemotherapy widely used in the management of high-grade glioma, including glioblastoma multiforme and anaplastic astrocytoma. Temoside 100 mg and Temoside 200 mg tablets are typically combined into individual daily doses as part of a carefully calculated regimen based on body surface area and treatment phase. Because temozolomide is taken by mouth, it offers the practicality of at-home administration, while still functioning as a full cytotoxic component of a neuro-oncology protocol that must be designed and monitored by experienced specialists.
In the standard Stupp protocol for newly diagnosed glioblastoma, temozolomide is used concurrently with radiotherapy and then continued as adjuvant cyclic therapy. Temoside temozolomide tablets fit into these patterns by providing mid- and higher-strength units that can be combined with other temozolomide strengths to reach the exact daily dose. The 1 pack / 5 tabs format is convenient for short cycles or specific dosing blocks, for example a 5-day schedule every 28 days, which is common in high-grade glioma regimens.
Generic Meds Mart supplies Temoside temozolomide tablets in original oncology packaging sourced only from licensed manufacturers and authorised distributors. Each blister strip and outer carton clearly shows the strength, generic name, batch number and expiry date, enabling hospital and clinic pharmacies to verify every pack. Parcels are shipped in neutral outer packaging with no external reference to brain tumour, cancer or chemotherapy, supporting patient privacy. Our role is limited to structured access and logistics; all decisions on whether, when and how to use Temoside temozolomide tablets remain with your neuro-oncology team.
Key Uses
Within the boundaries of local approvals and guidelines, Temoside temozolomide tablets are typically used for:
- Newly diagnosed glioblastoma multiforme, in combination with radiotherapy followed by adjuvant temozolomide cycles.
- Anaplastic astrocytoma and other selected high-grade gliomas, as part of multi-step regimens defined by neuro-oncology protocols.
- Recurrent or progressive high-grade glioma, where temozolomide may be used again or in adjusted schedules when clinically appropriate.
The precise indication, daily dose, cycle length and total number of cycles for Temoside 100 mg and 200 mg tablets are always determined by specialists who integrate imaging, histology, performance status and prior treatment history into the overall plan.
How Temozolomide Works in Chemotherapy
Temozolomide, the active ingredient in Temoside tablets, is an oral alkylating agent that exerts its anti-tumour effect by damaging DNA in rapidly dividing cells. After administration, temozolomide undergoes spontaneous conversion at physiological pH to an active compound that methylates DNA, particularly at the O6 and N7 positions of guanine. This methylation leads to mispairing during replication and triggers mismatch repair pathways that, when overwhelmed, result in apoptosis or cell death.
High-grade gliomas, including glioblastoma multiforme, are often infiltrative and difficult to control with surgery alone. By delivering temozolomide systemically, Temoside tablets help target malignant cells that remain after surgery and radiotherapy, as well as microscopic disease within the brain. The degree of benefit may be influenced by tumour molecular features such as MGMT promoter methylation status, which your care team may discuss when planning therapy. Temozolomide is not a cure for glioblastoma, but for many patients it provides improved survival and better disease control compared to radiotherapy alone.
Dosage & Administration
Temoside 100 mg and 200 mg tablets must only be prescribed and monitored by physicians experienced in neuro-oncology and chemotherapy. The total daily dose of temozolomide is usually calculated based on body surface area (mg/m²) and may vary between the concurrent chemoradiation phase and the adjuvant phase. Tablets of different strengths are combined to reach the exact daily dose, and schedules often use five consecutive days of dosing in a 28-day cycle during adjuvant therapy.
Temoside temozolomide tablets are typically taken once daily on an empty stomach, at least one hour before or two hours after food, with a glass of water. Tablets should be swallowed whole and not opened, crushed or chewed because the contents are cytotoxic. Antiemetic medications are commonly prescribed before each dose to help prevent nausea and vomiting. If vomiting occurs after a dose, patients should follow the instructions from their treatment centre and should not repeat the dose unless told to do so. All dose modifications, skipped doses and schedule changes must be directed by the oncology team.
Precautions
Before and during treatment with Temoside temozolomide tablets, careful monitoring is essential. Full blood counts are checked regularly to assess for neutropenia, thrombocytopenia and anaemia, with dose delays or reductions implemented if counts fall below protocol thresholds. Because temozolomide can suppress bone marrow, there is an increased risk of infections, including serious or opportunistic infections, and prophylactic measures such as Pneumocystis jirovecii pneumonia prophylaxis may be considered in some regimens.
Liver function tests are monitored for evidence of hepatotoxicity, and kidney function is also reviewed, especially in patients with comorbidities or concomitant medications. Patients should inform their team about any other medicines or supplements they are taking, since some drugs may influence bone marrow function, bleeding risk or nausea control. Temozolomide can harm an unborn baby, and effective contraception is recommended for both male and female patients during treatment and for a period after the last dose. Breastfeeding is generally not recommended while on Temoside temozolomide tablets.
Temozolomide Side Effects
Common side effects
Common temozolomide side effects reported with Temoside include nausea, vomiting, loss of appetite, fatigue, constipation, headache, hair thinning, mild rash, mild liver enzyme elevations and lowered blood counts such as neutropenia and thrombocytopenia. Many patients also experience general weakness or “flu-like” symptoms during or shortly after the 5-day dosing period.
These common temozolomide side effects can often be managed with pre-emptive antiemetics, adequate hydration, simple dietary adjustments, rest and supportive medications such as laxatives or pain relief as advised by the care team. Regular blood tests help the oncology team pick up falling blood counts early so doses can be adjusted and infections can be prevented or treated promptly. Patients should keep a record of symptoms between cycles and share this with their treating team.
Serious side effects
Serious temozolomide adverse effects require urgent evaluation and may include severe or prolonged neutropenia, febrile neutropenia, severe thrombocytopenia with bleeding, severe liver injury, serious opportunistic infections such as Pneumocystis pneumonia and rare secondary malignancies of the bone marrow. Warning signs such as high fever, chills, persistent cough, shortness of breath, easy bruising, nosebleeds, black or bloody stools, severe abdominal pain, yellowing of the skin or eyes and profound weakness should never be ignored.
If any of these symptoms occur while taking Temoside temozolomide tablets, emergency medical assessment is essential. The oncology team may need to stop or delay further doses, provide antibiotics, transfusions or other intensive supportive measures and reassess the overall chemotherapy strategy.
Storage
Temoside 100 mg and 200 mg temozolomide tablets should be stored in their original blister or bottle at the temperature specified in the package leaflet, generally below 25–30 °C, protected from moisture and direct heat. The pack should remain tightly closed when not in use and kept out of the sight and reach of children. Since temozolomide is cytotoxic, carers should follow local guidance for handling, including washing hands after touching the blister and avoiding contact with damaged tablets. Unused or expired Temoside tablets should be returned to a pharmacy or disposed of according to local cytotoxic waste procedures, not in household rubbish.
Why Buy from Generic Meds Mart
Generic Meds Mart focuses on structured access to essential oncology medicines such as Temoside temozolomide tablets. We work only with licensed manufacturers and authorised distributors who maintain Good Manufacturing Practice standards and provide full batch traceability. Each Temoside 100 mg and Temoside 200 mg pack is supplied in original sealed packaging so hospital and clinic pharmacies can verify authenticity, strength, batch number and expiry date.
Transparent USD pricing helps patients and institutions plan for multiple cycles of temozolomide, which may extend over several months of treatment. Neutral outer packaging and trackable international shipping, where regulations allow, support privacy and make it easier to coordinate deliveries with appointments and treatment schedules. Our role is strictly limited to access and logistics; all clinical aspects of temozolomide therapy remain under the control of your treating neuro-oncology team.
Order Now
Temoside temozolomide tablets are potent oral chemotherapy for high-grade glioma and must never be used without a personalised treatment plan from an experienced oncology service. Before arranging supply through Generic Meds Mart, ensure that your diagnosis has been confirmed, imaging and histology have been reviewed, and your team has decided on a temozolomide-based regimen including dose, number of cycles and monitoring schedule.
Once this plan is in place, your clinic or designated purchaser can calculate how many Temoside 100 mg and Temoside 200 mg packs (1 pack / 5 tabs) are required for the planned cycles, including any expected dose escalations or reductions. The necessary strengths and pack counts can then be selected on Generic Meds Mart, added to the cart and ordered through secure checkout in USD. Medicines will be dispatched in discreet packaging with original oncology labelling inside. Patients should never change, restart or stop temozolomide without explicit instructions from their neuro-oncology team.
FAQ about Temoside (Temozolomide)
Q1: What is Temoside used for?
Temoside contains temozolomide, an oral alkylating chemotherapy used mainly for high-grade glioma, including glioblastoma multiforme and anaplastic astrocytoma, in newly diagnosed and recurrent settings as part of structured neuro-oncology protocols.
Q2: Is Temoside a tablet or a capsule?
Temoside temozolomide in this presentation is supplied as oral tablets (1 pack / 5 tabs per strength). They are swallowed whole with water on an empty stomach and must not be opened, crushed or chewed because the contents are cytotoxic.
Q3: How long will I be on Temoside?
The length of treatment with Temoside temozolomide tablets varies depending on diagnosis, response and tolerance. Many glioblastoma protocols include a concurrent phase with radiotherapy followed by several adjuvant 28-day cycles. Your neuro-oncology team will explain how many cycles are planned initially and how this may change based on MRI scans, blood tests and side effects.
Q4: Will I lose my hair on temozolomide?
Temozolomide can sometimes cause hair thinning, but many patients experience much less hair loss than with some other chemotherapies. Because radiotherapy to the brain can also affect hair, your overall pattern of hair loss may be influenced by both treatments. Your team can advise what to expect in your specific regimen.
Q5: What monitoring will I need while taking Temoside?
Patients on Temoside temozolomide tablets usually require frequent blood tests to monitor white cells, neutrophils, platelets and haemoglobin, as well as liver function and, when relevant, kidney function. You will also be monitored for signs of infection, bleeding, severe fatigue or neurological changes, and regular brain imaging will be scheduled to assess treatment response and disease status.
Be the first to review “Temoside Temozolomide – 100 mg, 200 mg Tablets (1 pack / 5 tabs)” Cancel reply
Related products
Anticancer






Reviews
There are no reviews yet.